YUPELRI delivers a full 24 hours of efficacy in a single, nebulized, daily dose1
YUPELRI was studied in two 12-week, randomized, double-blind, placebo-controlled, parallel-group confirmatory studies (Studies 1 and 2) to evaluate the efficacy of once-daily YUPELRI vs placebo in patients with moderate to very severe COPD
Consistent improvement in FEV1 vs placebo over 24 hours on days 84/851,11
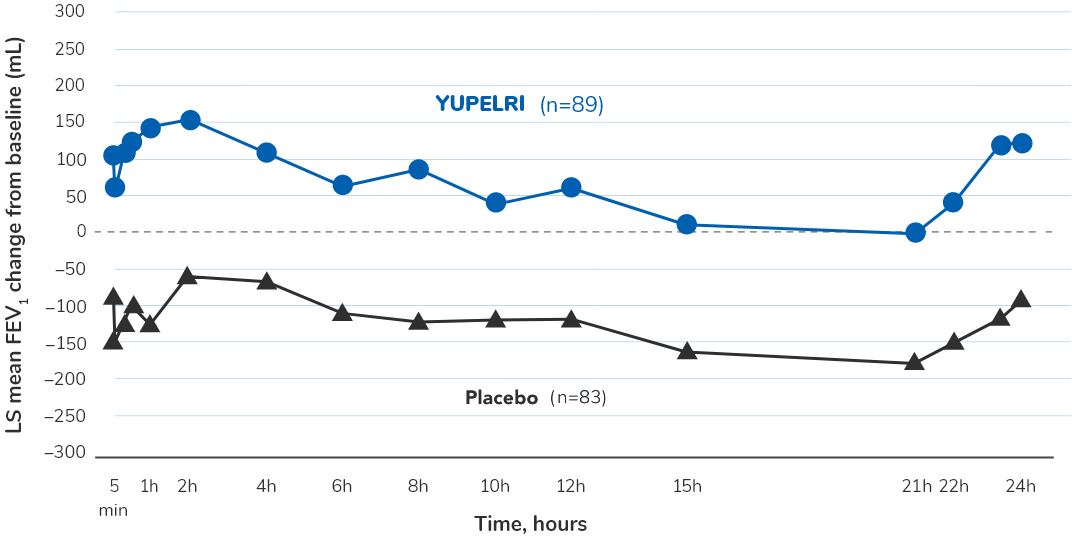
In Studies 1 and 2, serial spirometry was performed on a substudy population. Pooled results are shown. Primary efficacy endpoint was change from baseline in trough (predose) FEV1 at day 85 vs placebo.
- In Studies 1 and 2, a prespecified exploratory analysis was performed. In Study 1, LS mean changes from baseline in FEV1 ranged from 55.8 mL to 240.4 mL in the YUPELRI group, and from -113.6 mL to 59.6 mL in the placebo group. In Study 2, LS mean changes from baseline in FEV1 ranged from 19.8 mL to 148.5 mL in the YUPELRI group, and from -176.4 mL to -13.0 mL in the placebo group11
LS=least squares.



